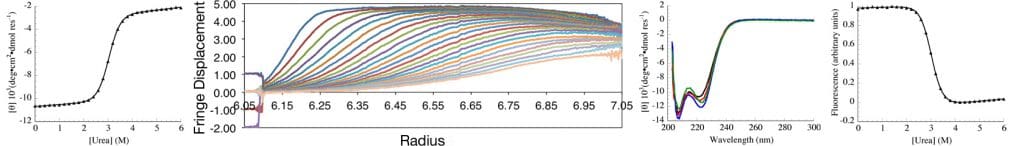
The Center for Molecular Biophysics (CMB) at the Krieger School of Arts and Sciences (KSAS) is a partnership between faculty in the departments of biology and biophysics and the Krieger School administration, with faculty generously contributing state-of-the art solution biophysics instrumentation and KSAS contributing toward operations.
The goal of CMB is to provide the resources and intellectual environment to facilitate biophysical research, both for biophysically oriented laboratories, and for those laboratories whose research has not traditionally involved structural or biophysical characterization. To facilitate this goal, the center provides three resources: instrumentation, project development, and education.